beREADY - endometrial receptivity test

Benefits of endometrial receptivity test
71.2%
Did you know that 71.2% of patients gave birth after the first year of IVF treatment using the endometrial receptivity test*?
72.5%
Please note that 72.5% pregnancy rate was achieved by the first IVF cycle when endometrial receptivity test was used*
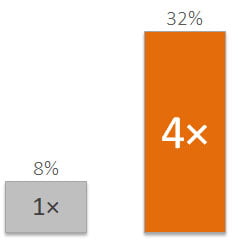
Have you experienced IVF failure?

Did you know that 1 out of 3 patients experience IVF failure because the embryo is transferred too early or too late?****
What does personalised medicine mean in IVF?

What does the beREADY test show?
The test measures the maturity level of your endometrium. The information you get enables embryo transfer at the optimal time. The results may show three possible scenarios with probability percent. 42% of beREADY patients have pre- or early-receptive diagnose and they need more time to mature. About half of the patients have an "average" maturation speed, and approximately 10% need less time for maturation compare to "average". As you see it is difficult to make a diagnosis without testing
42%
PRE or EARLY-RECEPTIVE
You need more time! The test shows that you have shifted WOI, and embryo transfer should be done later. So, the report indicates the exact timing that is optimal for you
49%
FULLY-RECEPTIVE
The test confirms that you have an average and expected WOI and there is no need to change the timing
9%
LATE or POST-RECEPTIVE
You have rapid endometrial maturation! The test shows that you have shifted WOI, and embryo transfer should be done earlier. So, the report indicates the exact timing that is optimal for you
How does the beREADY test work?
Endometrial sample
Sample shipping to beREADY lab
Sample processing and quality control
beREADY analysis and reporting
Embryo transfer at the right time
Results are available in 1-2 weeks
Comparison of endometrial receptivity tests
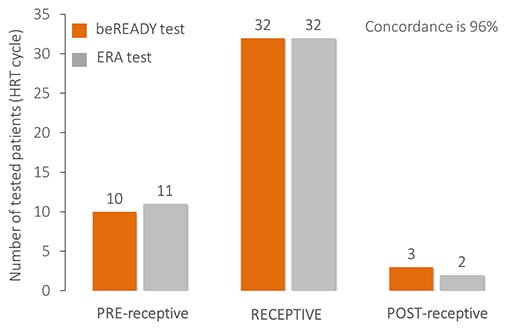
The beREADY test is developed based on carefully classified samples and independent clinical validation.
As a comparison, 45 HRT patients’ samples were involved in a comparison against the Endometrial Receptivity Analysis (ERA, Igenomix) test. The concordance rate between the two tests is as high as 96% because the same tissue samples were analyzed.
The beREADY test applies novel and highly accurate TAC-seq DNA sequencing technology. The technology enables the detection of biomarker molecules at a single-molecule level and provides ultimate sensitivity.


beREADY test uses highly sensitive and patented TAC-seq technology because only that approach ensures accuracy. The original data analysis algorithm is published*** and adjusted later to beREADY diagnostic assay.
beREADY test is a CE-IVD medical device. It is controlled, validated, and licensed to distribute in European Union.
Referred publications and research behind beREADY test
* Simón et al., A 5-year Multicenter Randomized Controlled Trial of In Vitro Fertilization with Personalized Blastocyst Transfer versus Frozen or Fresh Transfer. 2020, Reproductive BioMedicine Online, PubMed ID 32723696. Link to original study
** Haouzi et al., Customized Frozen Embryo Transfer after Identification of the Receptivity Window with a Transcriptomic Approach Improves the Implantation and Live Birth Rates in Patients with Repeated Implantation Failure. 2021, Reproductive Sciences, PubMed ID 32725589. Link to original study
*** Teder et al., TAC-seq: targeted DNA and RNA sequencing for precise biomarker molecule counting. 2018, npj Genomic Medicine, PubMed ID 30588329. Link to original study
**** Ruiz-Alonso et al., The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. 2013, Fertility and Sterility, PubMed ID 23756099. Link to original study
Saare et al., A molecular tool for menstrual cycle phase dating in endometriosis transcriptomic studies. 2019, Biology of Reproduction, PubMed ID 31004479. Link to original study
Suhorutshenko et al., Endometrial receptivity revisited: endometrial transcriptome adjusted for tissue cellular heterogeneity. 2018, Human Reproduction, PubMed ID 30295736. Link to original study
Altmäe et al., Meta-signature of human endometrial receptivity: a meta-analysis and validation study of transcriptomic biomarkers. 2018, Scientific Reports, PubMed ID 28855728. Link to original study
Krjutškov et al., Single-cell transcriptome analysis of endometrial tissue. 2016, Human Reproduction, PubMed ID 26874359. Link to original study




